Within the Winsmart project, different technologies of optically
switchable windows are being developed. The "state of the art"
electrochromic window with oxide electrochromic and oxide counter
electrode, separated by an ion conductor will be further
improved.
As alternative, electrochromic windows with redox electrolyte
are being developed.
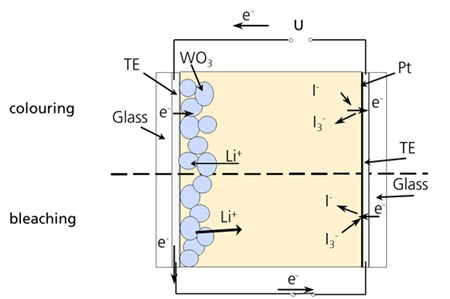
Schematic configuration of an electrochromic device with
WO3 as electrochromic layer on a transparent electrode
(TE) in contact with a redox electrolyte comprising Li+,
I- and I3- ions. The counter
electrode is realized by sputtering a very thin layer of platinum
on a second transparent electrode.
Here, an electrochromic layer is deposited on a transparent
conducting electrode (TE) being in contact with a redox electrolyte
comprising for example Li+, I- and
I3- ions, I-and
I3- acting as redox couple.
The counter electrode is prepared by sputtering a very thin
layer of platinum as a catalyst on another TE. Under negative
polarization, electrons are shifted from the counter electrode,
where I- is oxidized to I3-, to
the electrode with WO3, where Li+ ions are
injected out of the electrolyte, which leads to a coloration. For
bleaching, the polarization is reversed. By this, high optical
contrasts can be achieved. Especially, colourless redox couples can
be applied instead of the I-/I3-
couple, which may create a pale yellow. Therefore, high
transparencies can be reached.
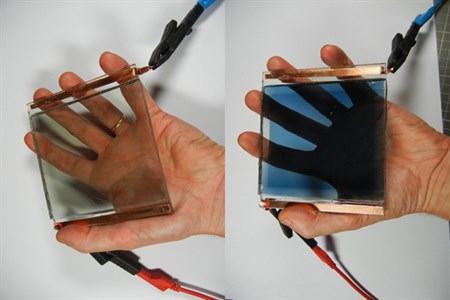
Electrochromic device with transparent redox electrolyte in
bleached (left) and coloured state (right).
Another development closely related to the electrochromic device
with redox electrolyte is the photochromic device based on a
combination of a dye solar cell and electrochromic tungsten
oxide.
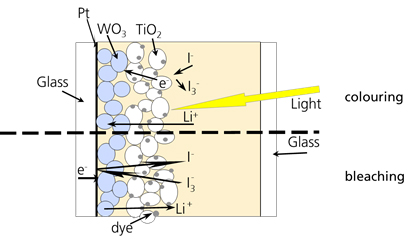
Schematic configuration of a photochromic device with
WO3 as an electrochromic layer, TiO2 and dye
as photoactive layer, in contact with a redox electrolyte.
Here, a dye is adsorbed on a porous surface of a layer out of
TiO2, which is in contact with an electrochromic layer
of WO3. Upon illumination, the dye is excited, and the
excited electron is transferred to the WO3 via the
TiO2, creating a deep blue colour. In parallel, the dye
is reduced to the ground state by oxidation of I- ions,
forming I3-, and Li+ ions are
intercalated into WO3 out of the redox electrolyte. The
bleaching process is realized by adding a catalyst like Pt, which
catalyzes the back reaction of the electrons from WO3 to
I3- in the redox electrolyte. In contrast to
previous works based on sol-gel chemistry, now at Fraunhofer ISE it
has been achieved to prepare photoelectrochromic devices by
sputtering.
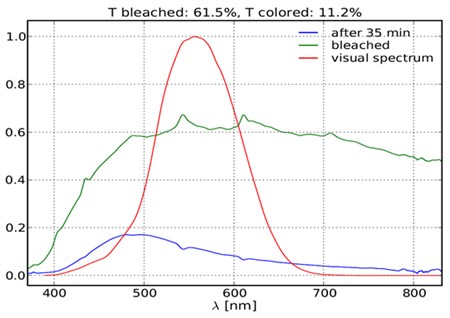
Transmittance spectra of sputtered photoelectrochromic
device in bleached and coloured state (upon illumination).

Sputtered photoelectrochromic device in bleached and
coloured state (upon illumination).
Furthermore, at the University of Ljubljana, it was
possible to prepare a photochromic coating based on a special
solgel-synthesis of tungsten oxide without redox electrolyte or dye
on TiO2, which colours upon illumination and bleaches in
the dark.

Photochromic WO3 layers without dye and redox
electrolyte in bleached (left) and after colouring by illumination
for 60 min (right).

This project has received funding from the European
Union Seventh Framework Programme (FP7/2007-2013) under grant
agreement n° 314407.